Asqelio® intraocular lens

Operation Steps

Model:MY18
Medical Device Registration Certificate Number:NMPA Registration No.20253160119

Asqelio®intraocular lens is a pale-yellow one-piece posterior chamber lens, foldable, modified C-loop haptics, aspheric monofocal optical design, used foraphakia correction after cataract extraction in adults. The optical part and the supporting part are made of hydrophobic acrylic material, adding ultraviolet absorbent and blue light filtering properties . The filtering ability of wavelength 400~475nm is similar to that of young human lens, which can effectively reduce the damage caused by blue light radiation, not only can protect the retina but also do not affect the night vision. The product is sterilized by ethylene oxide and single-use.
Special material
- Glass transition temperature of 11℃ , shows excellent soft features, is advantageous to the minimally invasive surgery using.
- Stable material, with high quality cutting process at low temperature, no glistening phenomenon.
Precise mechanism design
- 360 ° square edge and the vertical plane design, effectively prevent the occurrence of secondary cataract (PCO)
- Improved C-loop design provides excellent supporting ability, maintaining for a long time in the centration. Good elasticity of the material provides easy folding and unfolding during the surgery. Surgical wound can be decreased to only 2.2 mm
Excellent optical performance
- -0.27 um spherical aberration of aspheric optical design, with high color contrast, either during the day or night, Asqelio® Intraocular Lens can provide the best visual quality
- Abbe number 50 of the material can reduce the dispersion effect, and reduce all kinds of color in the visual disturbance, providing better visual quality for the patients
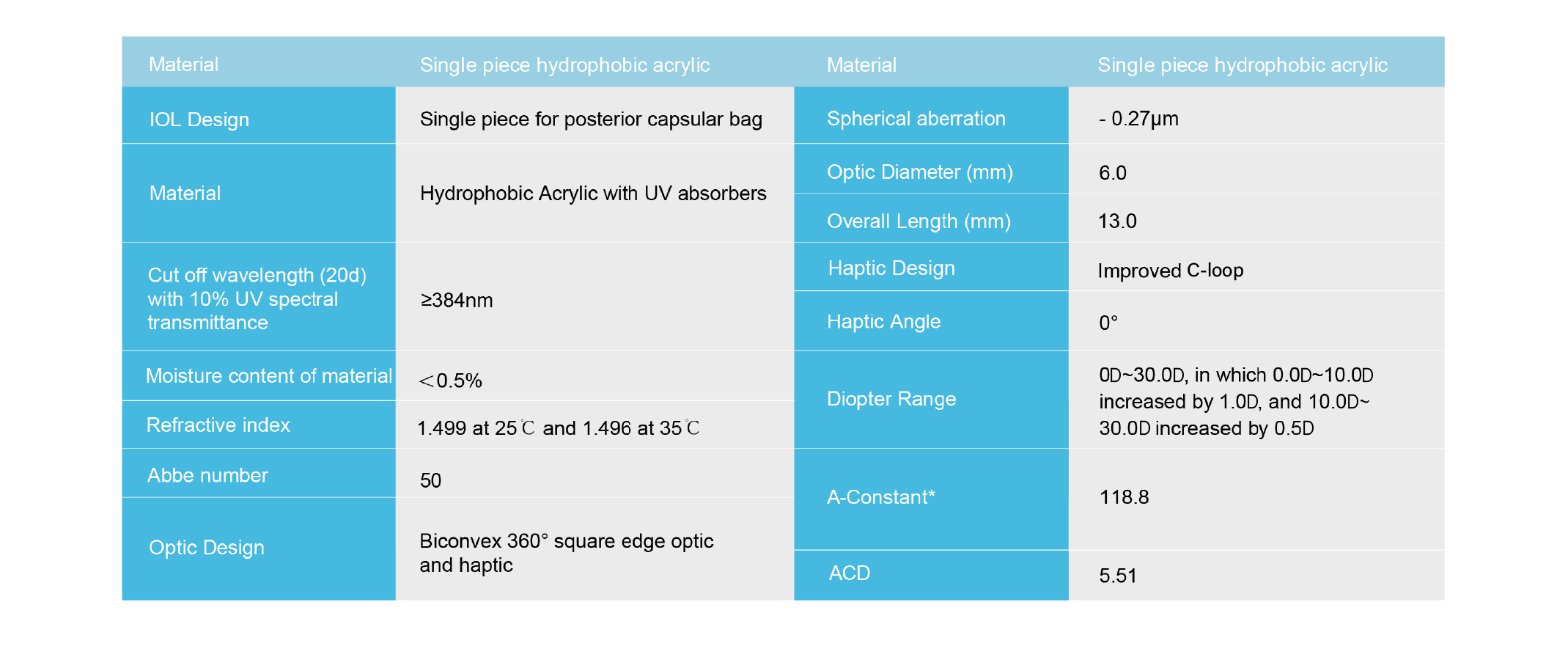
Main performance parameters of the product

Asqelio® Preloaded IOL Delivery System
Model:MY18PC, MY18PD
Medical Device Registration Certificate Number:NMPA Registration No.20243161602

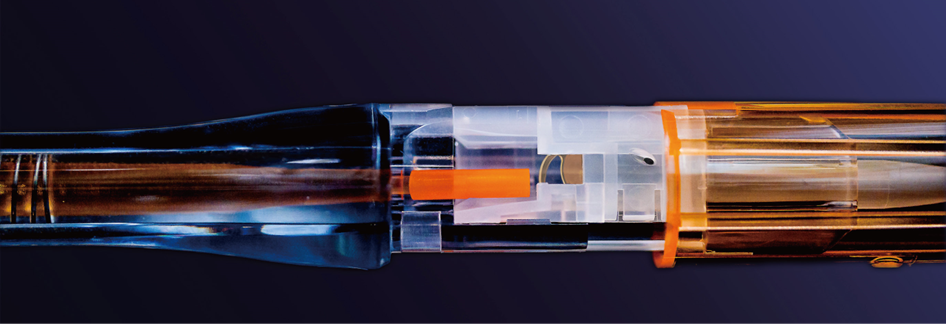
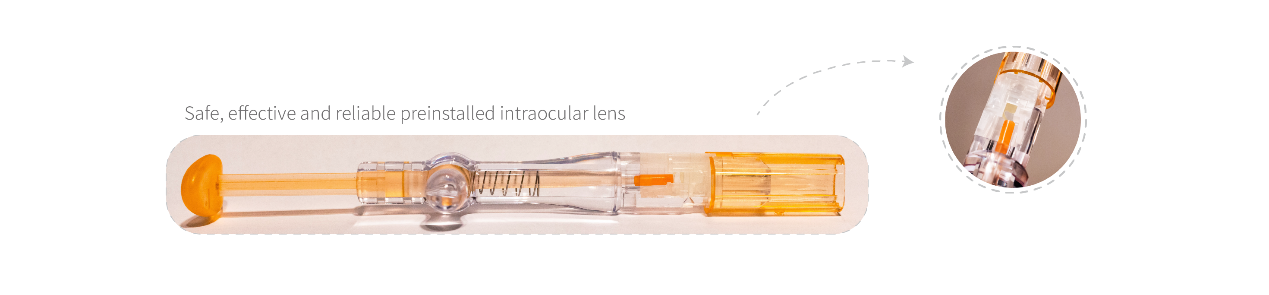
Asqelio® Preloaded IOL Delivery System
Consists of an intraocular lens and an injector. The Asqelio® intraocular lenses is pre-installed in the injector. After phacoemulsification, the intraocular lens can be simply implanted into the eye, eliminating the complex operation of installing the lens during the surgery. According to the size of the injector tip, we have two models: MY18PC and MY18PD, which are respectively suitable for surgical incisions as small as 2.6mm and 2.2mm. This product is sterilized by ethylene oxide and is for single-use only.

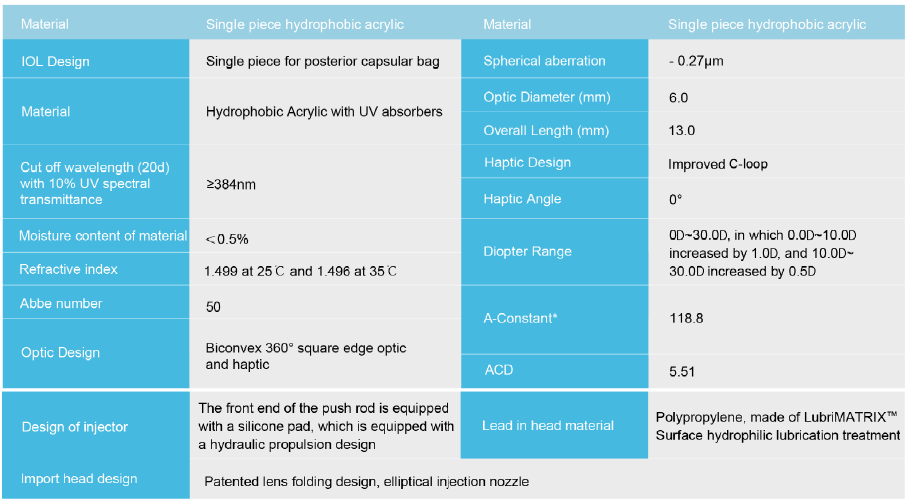
Main performance parameters of the product

Operation Steps

