- lioli® IOL Delivery Devices
Model:BF01,BF02
Manufacturer:Shuochuang (Shanghai) Medical Equipment Co., LTD
Medical device registration number:Shanghai Registration NO.20242160032

「lioli® IOL Delivery Devices」
BF series IOL Delivery Devices is a very cost-effective product, hydraulic propulsion is designed to facilitate one-handed operation during surgery. The cartridge has a built-in rail design to facilitate easy loading of the intraocular lens. The surface is treated with AST's proprietary LubriMATRIX™ hydrophilic lubrication coating. Good biocompatibility and stability is the feature of the coating. Feel confident to use this product in cataract intraocular lens implantation. Not only makes the push of the IOL smoother but also make sure that no other ingredients are implanted in the eye.
Product Model
BF IOL Delivery Devices have 2 models: BF01 and BF02. It is suitable for intraocular lens implantation with surgical incision as small as 2.2mm and 2.6mm respectively. The circular design of the front tip can avoid the IOL surface crease and crush injury during the injection process. Enabling a wider range of suitable IOLs. This product is sterilized by ethylene oxide. A box of 10 sets, each set is fitted with a single-use sterile intraocular lens implantation system.

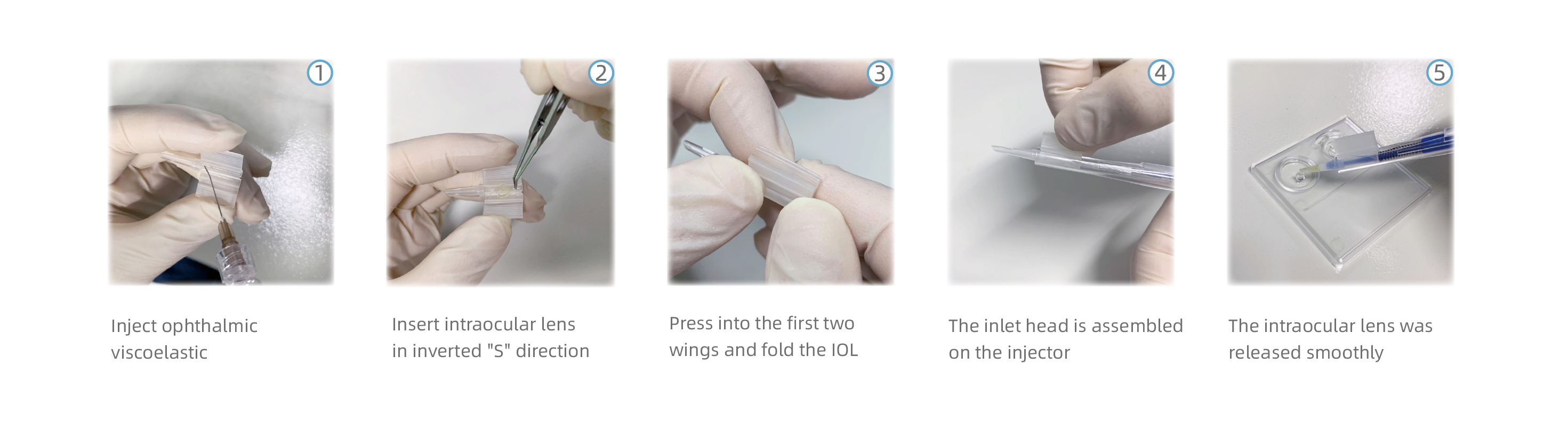
Operation Steps